Exploring the Science Behind the Metallic Taste of Blood

Blood, the life-sustaining fluid flowing through our veins, harbors mystery and intrigue. Although the taste of blood is not an everyday experience for most people, those who have had the misfortune to encounter it often describe a distinct metallic taste. In this article, we will embark on a scientific journey to understand why blood tastes metallic by examining the intricacies of its composition and sensory perception.
Composition of Blood
To understand the metallic taste of blood, you must first study its composition. Blood consists of several components, including red blood cells, white blood cells, platelets, plasma, and a complex set of organic and inorganic substances. Of particular interest is the presence of iron, which plays a vital role in the function of hemoglobin, the molecule responsible for oxygen transport.
The composition of blood is a complex and fascinating topic. Blood is made up of various components that work together to perform essential functions in the body. Here is a more detailed overview of the major components of blood:
- Red blood cells (RBCs): Red blood cells, also known as red blood cells, are the most numerous cells in the blood. Their main role is to transport oxygen from the lungs to all body tissues and to carry carbon dioxide back to the lungs for removal. RBCs contain the protein hemoglobin, which binds to oxygen and gives the blood its characteristic red color;
- White blood cells (WBCs): White blood cells, or white blood cells, are responsible for protecting the body from infection and disease. There are different types of white blood cells, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils. Each type plays a role in the immune response, such as absorbing pathogens, producing antibodies, or coordinating immune responses;
- Platelets: Platelets, also called thrombocytes, are small fragments of cells involved in blood clotting. When a blood vessel is damaged, platelets adhere to the site of damage and release clotting factors that trigger a cascade of reactions that result in the formation of a clot, preventing heavy bleeding;
- Plasma: Plasma is the liquid component of blood and makes up about 55% of its total volume. It is a yellowish fluid consisting mainly of water, but also various proteins, electrolytes, hormones, breakdown products, and nutrients. Plasma plays an essential role in transporting substances through the body, maintaining blood pressure, and regulating body temperature;
- Organic and Inorganic Substances: In addition to cellular and plasma components, blood contains various organic and inorganic substances. One important component is iron, which binds to hemoglobin in red blood cells and provides oxygen transport. Other important organic substances found in the blood include glucose, lipids, amino acids, hormones, and enzymes.
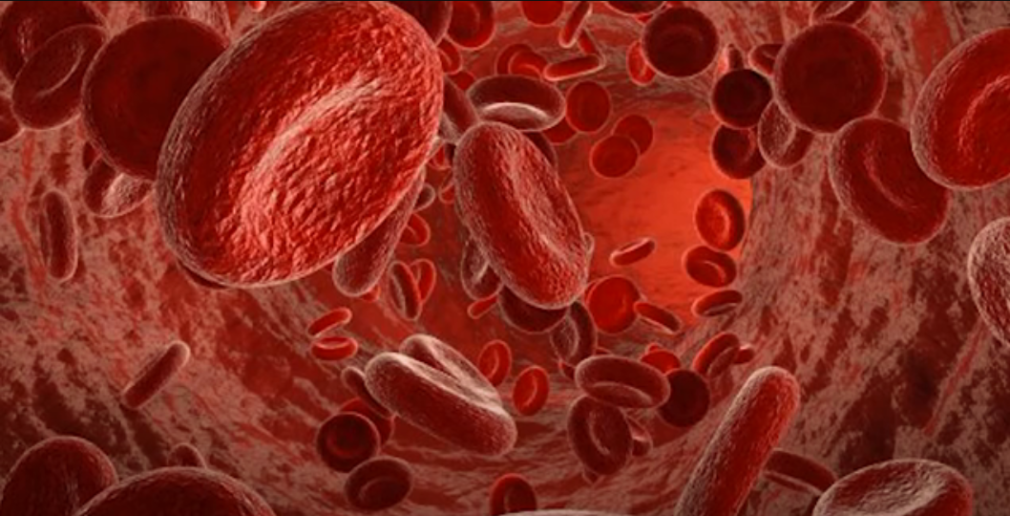
Overall, the complex composition of blood allows it to perform vital functions such as carrying oxygen, immune defense, blood clotting, and transporting nutrients and waste products throughout the body.
Iron and Metal Taste
The metallic taste associated with blood is primarily due to the presence of iron in it. When blood comes into contact with taste buds on the tongue, specific proteins bind to iron ions, initiating a chemical reaction. This interaction causes the release of electrons from the metal, resulting in an electric current. Iron plays an important role in the perception of the metallic taste associated with blood.
Here is a more detailed look at the relationship between iron and metallic taste:
- Blood Iron: Iron is an important mineral found in the hemoglobin portion of hemoglobin, the molecule responsible for oxygen transport in red blood cells. Each hemoglobin molecule contains four iron ions that bind to oxygen molecules. This binding and release of oxygen are crucial for the exchange of gases between the lungs and body tissues;
- Interaction with taste buds: When blood comes into contact with taste buds on the tongue, a chemical reaction occurs. Specific proteins in saliva, called chelators or metal-binding proteins, bind to iron ions present in the blood. This interaction triggers a series of reactions that result in the release of electrons from iron ions;
- Perception of metallic taste: Electrical signals produced by the interaction of iron ions with taste receptors are transmitted to the brain. These signals are interpreted by the brain as metallic taste sensations. The unique combination of iron ions, proteins, and enzymes in the blood creates a taste profile that has a distinct metallic taste.
Individual variations: It is important to note that not everyone perceives the metallic taste of blood in the same way. Differences in taste sensitivity, genetic factors, and previous sensory experiences can affect how a person perceives blood taste. Factors such as medications, dietary components, and general oral health can also affect taste perception.
Evolutionary Implications
The ability to detect and recognize the metallic taste of blood has important evolutionary implications. This innate response is thought to have evolved as a defense mechanism against the consumption of harmful or contaminated substances. In both humans and animals, the metallic taste of blood is associated with potential dangers, such as injury or the presence of toxins. This primal reaction triggers heightened awareness and caution, promoting self-preservation.
Variations in Perception
Although most people describe the taste of blood as metallic, slight variations in perception are possible. Factors such as a person’s sense of taste, genetic predisposition, and previous sensory experience may influence the perception of blood. Some people may experience a stronger metallic taste, while others may have a more muted taste. In addition, the presence of other substances, such as medications or dietary factors, can also affect taste perception.
Conclusion
Blood metal taste is due to a complex interaction between iron ions and taste buds on the tongue. This sensation has deep evolutionary roots as an early warning system for potential threats. By uncovering the scientific background of blood taste, we gain a deeper understanding of the complex nature of our senses and the amazing ways in which our bodies have adapted to survive.
So the next time you encounter the unique taste of blood, remember that it is evidence of the complex workings of our physiology and the fascinating world of hematology.